|
Article Information
|
Authors:
Khumbulani W. Hlongwana1
Alpheus Zitha2
Aaron M. Mabuza2
Rajendra Maharaj1
Affiliations:
1Malaria Research Programme, South African Medical Research Council, Durban, South Africa
2Malaria Control Programme, Department of Health and Social Services, South Africa
Correspondence to:
Khumbulani Hlongwana
Email:
khlongwana@mrc.ac.za
Postal address:
South African Medical Research Council, 491 Ridge Road, Overport, Durban, 4001, South Africa
Dates:
Received: 25 Nov. 2010
Accepted: 12 Feb. 2011
Published: 12 July 2011
How to cite this article:
Hlongwana KW, Zitha A, Mabuza AM, Maharaj R. Knowledge and practices towards malaria amongst residents of Bushbuckridge, Mpumalanga,
South Africa. Afr J Prm Health Care Fam Med. 2011;3(1), Art. #257, 9 pages.
doi:10.4102/phcfm.v3i1.257
Copyright Notice:
© 2011. The Authors. Licensee: AOSIS OpenJournals. This work is licensed under the Creative Commons Attribution License.
ISSN: 2071-2928 (print)
ISSN: 2071-2930 (online)
|
|
|
|
Knowledge and practices towards malaria amongst residents of Bushbuckridge, Mpumalanga, South Africa
|
|
In This Original Research...
|
Open Access
|
• Abstract
• Introduction
• Setting
• Significance of the study
• Methods
• Setting
• Design and data collection
• Analysing
• Results
• Characteristics of study population
• Knowledge of malaria and information, education and communication
• Treatment-seeking behaviour and prevention
• Indoor residual spraying
• Discussion
• Knowledge of malaria
• Malaria information, education and communication
• Treatment-seeking practices in terms of promptness and treatment options
• Personal protection against malaria and indoor residual spraying
• Conclusion
• Acknowledgements
• Competing interests
• Authors’ contributions
• References
|
|
Background: Malaria remains one of the greatest public health challenges worldwide and it is amongst the top killers in sub-Saharan Africa.
There is however, a general scepticism about the accuracy of Health Management Information Systems (HMIS) in recording all the episodes of malaria
in Africa. Given the importance of community knowledge of malaria, its signs and symptoms, as well as prompt treatment-seeking behaviour, the study
assessing adult residents’ knowledge and practices in Bushbuckridge provided much needed insights into the Malaria Control Programme (MCP).
Objectives: The objectives of this study were to determine the adult residents’ knowledge and practices towards malaria in Bushbuckridge,
Mpumalanga Province, South Africa.
Method: The study was undertaken as a descriptive cross-sectional survey in Bushbuckridge in August 2008. Six hundred and two (602) household
heads or their proxies from the randomly selected households in 20 localities were interviewed (one household member per household), using a structured
field-piloted questionnaire.
Results: Approximately 93% of the respondents had heard about malaria, 84.6% of whom correctly associated it with mosquito bites. The health
facility (29.1%) and radio (19.8%) were the main sources of malaria information. Knowledge of signs and symptoms was low, whilst treatment-seeking
intention at the health facility was high (99%) with 82% of which would be carried out promptly. Survey data showed an indoor residual spraying (IRS)
coverage of approximately 70% and a good understanding of the reasons for spraying. Walls were re-plastered infrequently and no evidence was established
linking it to the removal of insecticide marks on the wall.
Conclusion: The study revealed not only that householders possessed an adequate knowledge of malaria, but also that they had positive malaria
treatment-seeking intentions. Their knowledge of malaria signs and symptoms was inadequate and required attention. Whilst IRS coverage needed some
improvements, the reasons for IRS were well known.
Setting
Malaria remains one of the world’s greatest public health challenges,1 killing over one million people per
year,2 and up to 500 million clinical malaria cases occur globally.3,4 Whilst Africa accounts for over 90% of
the disease burden worldwide,5,6,7,8,9 sub-Saharan Africa is the worst afflicted malaria region,10,11,12,13 and
malaria is one of the top killers.14 Other authors8 insist that malaria episodes in Africa are underestimated
and many cases never reach health facilities, and are therefore not captured by the health management information systems (HMIS). Notably,
the flow of malaria cases to health facilities is likely to be affected by the treatment-seeking behaviour as well as the amount and
quality of malaria health education provided to the community. Human migration across national borders is another critical factor which
is likely to compromise the effectiveness of malaria-control interventions,15,16 especially malaria information, education and
communication (IEC) and promptness in treatment-seeking practices. Cross-border movement does not only pose a risk of malaria parasites
being imported into the country, but some people may arrive after IEC activities have been conducted, so the monitoring of IEC impact
can become very difficult.
Despite the documentation of numerous health compromising factors, some studies have emphasised the value of adequate knowledge of malaria in
order to ensure that people apply preventive measures, and seek prompt and appropriate treatment for themselves and their dependants.9,17
It is necessary, therefore, that people’s knowledge and practices with regard to malaria is regularly assessed and promoted. Nevertheless,
no malaria knowledge and practices studies were previously conducted in Bushbuckridge. The main rationale for this study was to investigate and
describe the local adult residents’ understanding of malaria transmission, their recognition of signs and symptoms, their perceptions of
cause, treatment and preventive practices, and indoor residual spraying (IRS), in view of the migratory trend between Mozambique and Bushbuckridge.
The ultimate goal was to produce informative results which could be used to improve the educational materials designed for use in IEC intervention strategy.
In South Africa, malaria transmission is seasonal and restricted to the north-eastern border areas with Mozambique, Swaziland and Zimbabwe.18,19
Approximately 4.9 million (10%) of South Africa’s total population are at risk of contracting malaria20 and annual national
malaria cases were estimated at 32 530 in the year 2006.21 Anopheles arabiensis remains the only confirmed vector plaguing
the country; following the elimination of Anopheles funestus through years of IRS with dichlorodiphenyltrichloroethane (DDT).19
Plasmodium falciparum is the most prevalent parasite, accounting for approximately 95% of all malaria cases in South Africa.22
IRS with DDT and K-Othrine (a pyrethroid insecticide), complemented with effective case management (definitive diagnosis and effective treatment),
disease surveillance and malaria health promotion are the major control strategies employed in Bushbuckridge. IRS is implemented once a year, whereby
K-Othrine is applied on smooth-surface painted inner house walls (usually referred to as western structures) and DDT is applied on rough-surface
unpainted inner house walls (usually referred to as traditional structures). Artemisinin combination therapies (ACT) and quinine remain the first
and second-line treatment drugs, respectively.
Significance of the study
Malaria in South Africa remains a public health challenge as in various other countries of the world. There are two striking factors about
this study, that is, the timing of the study and the geographical location of the study site. The timing of the study is significant, because
it was carried out after South Africa was declared ready to eliminate malaria. Geographically, the study area is in close proximity to Mozambique,
which is still in the control stage of malaria, whilst there is documented evidence of high cross-border movements between Mozambique and Bushbuckridge.
Most importantly, results of the study were to be used directly by the Malaria Control Programme to improve its intervention strategies, especially with
regard to malaria information, education and communication (IEC).
Setting
Mpumalanga Province, the third smallest populated province in South Africa, occupies 6% of the country’s surface area and lies
between Mozambique in the east, Swaziland and KwaZulu-Natal Province in the south, Limpopo Province in the north and Gauteng Province
in the west.23 Bushbuckridge subdistrict comprises 67 localities (villages) that are clustered into 10 Sectors as demarcated
by the Malaria Control Programme for operational purposes (Figure 1).25 It extends over an area of 2 400 km2,25
is populated by approximately 700 000 people and has more than 110 000 households.21,25,26 Approximately 30% of the adult population
originate from Mozambique,27 the majority of whom have settled in Bushbuckridge during the Mozambican civil wars of the 1980s.28,29
The Shangaan (vernacular) language and culture shared by the migrants and the local inhabitants, played a paramount role in Mozambican
migrants’ choice of Bushbuckridge.29
 |
FIGURE 1: The location of study sites in Bushbuckridge and the position of the subdistrict within the country.
|
|
Polzer28 has documented the systematic integration of Mozambican refugees in Bushbuckridge extensively, as well as regular cross-border
movements, from a social-connectivity point of view. From a malaria-epidemiological point of view, however, cross-border human movement provides a
critical dimension, given the geographical proximity of Mozambique to South Africa and the frequency of cross-border movements. The upsurge of malaria
cases in Bushbuckridge during the 2009–2010 malaria season is a cause of major concern, given the maturing efforts to eliminate malaria in South
Africa (Figure 2).30 Another important dimension is the two countries’ positions in the malaria-control continuum. South Africa, for
example, is already preparing for malaria elimination after being earmarked and declared by the African Union (AU) and the Southern Africa Development
Community (SADC) as one of the countries ready for malaria elimination, alongside Swaziland, Botswana and Namibia.31,32 Mozambique, on the
other hand, is still at the control stage of malaria.
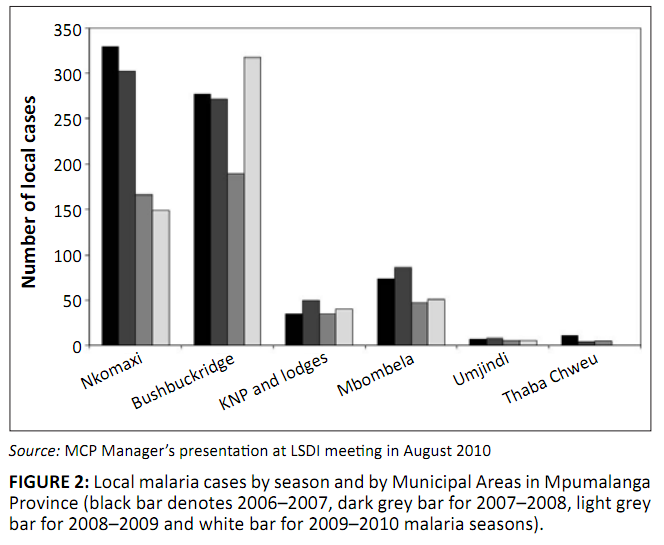 |
FIGURE 2: Local malaria cases by season and by Municipal Areas in Mpumalanga
Province (black bar denotes 2006–2007, dark grey bar for 2007–2008, light grey
bar for 2008–2009 and white bar for 2009–2010 malaria seasons).
|
|
It appears that imported cases will continue to pose a serious challenge to malaria- control efforts in South Africa and Bushbuckridge in particular.
This assertion is confirmed by the recorded trend of malaria cases in Mpumalanga Province (Figure 3),32 whereby, over time, the number of
imported cases has exceeded that of local cases. There is already documented evidence elsewhere that confirms that the stream of incoming malaria cases
from the neighbouring countries compromise the effectiveness of malaria-control interventions.15 The complexity of the cross-border movements
in Bushbuckridge is further entrenched through intermarriages, a phenomenon that has implications on malaria-control interventions, especially on the eve
of a malaria-elimination programme in South Africa.
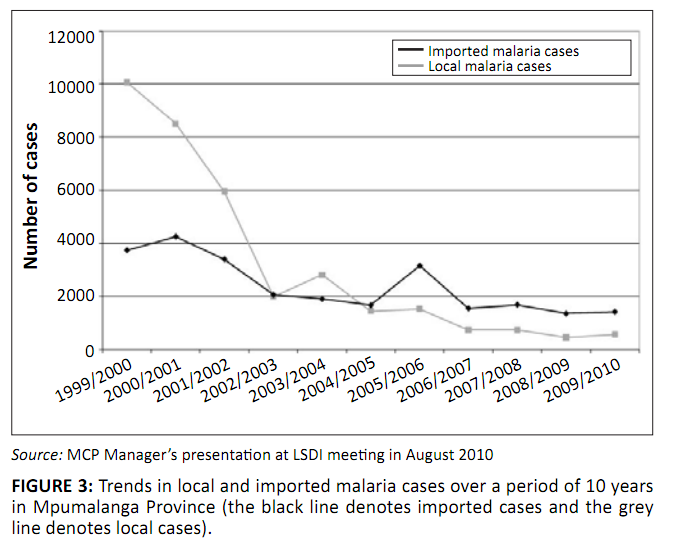 |
FIGURE 3: Trends in local and imported malaria cases over a period of 10 years
in Mpumalanga Province (the black line denotes imported cases and the grey
line denotes local cases).
|
|
Design and data collection
The study was conducted as a descriptive cross-sectional survey. Six locally recruited and competently trained fieldworkers administered a
standardised pretested structured questionnaire to 602 adult respondents over a period of 2 weeks (August 2008). The questionnaire (translated
from English to XiTsonga and SePedi) included closed-ended, partially closed and open-ended questions (see Appendix B: Questionnaire in English).
Study participants were household heads and/or their proxies older than 18 years.
If it is assumed that the number of households has increased from 110 000 to 130 000, a sample size can be calculated based on the systematic
conformal sampling technique (refer to the following equations), using the error margin of 4% and the confidence level of 95%, to result in a
minimum sample size of 598 households.
x = Z (c/100) 2r (100 - r) [Eqn 1]
n = Nx/([N - 1] E2 + x) [Eqn 2]
E = √([N - n] x/n [N - 1]) [Eqn 3]
The variables are: n, the sample size; N, the population size; E, the margin of error; r, the fraction of the response
that we are interested in (with a normal distribution of 50%), and Z(c/100), the critical value for the confidence level.
The overall sampling strategy was a multistage process, whereby two localities were randomly selected in each sector before disproportionately
identifying approximately 30 households (one participant per household) in each locality. Households were identified through systematic sampling
with a random start, whereby every tenth household was selected until the required sample size was acquired. Ethics approval was granted by the
Mpumalanga Provincial Research and Ethics Committee.
Analysing
Survey data were entered directly into an electronic data collection system using Microsoft Access, and checked for accuracy prior to analysis.
Data were imported from Access, and analysis was performed using the Epi-info (version 3.3.2) statistical software programme. Firstly, a descriptive
statistical analysis was carried out. A bivariate analysis (chi-squared test) was applied to compare proportions and to generate Odds Ratios. The
statistical significance of the association between variables was assessed using 95% Confidence Intervals (CI). In addition to textual descriptions,
results were summarised in the form of Tables and Figures.
Characteristics of study population
Three-quarters of the heads of households or their proxies who participated in the study were female (n = 451, 75.0%). Approximately
one-third of the respondents were under 30 years old, with the average median ages of 35 (s.d. = 14) and 38 years (s.d. = 17) for female and
male participants respectively. A number of households (n = 262, 43.5%) had family sizes of 4–6 members. Fewer households
(n = 32, 5.3%) had family members exceeding nine, whilst households with 1–3 and 7–9 family members were 27.1% (n = 163)
and 24.1% (n = 145), respectively. More than half of the respondents (58.8%) had completed secondary education, whilst 20.6% had no formal
education. About two-thirds of the respondents were either unemployed or housewives. Self-reported data showed that 11.5% of the respondents had
been tested for malaria within the 12 months prior to the survey. Reasons for undergoing malaria tests, the results of the tests and testing facilities
were not investigated. Few (4.2%) of the respondents were reportedly infected with malaria within the period under investigation. Detailed
characteristics of the respondents are presented in Table 1.
|
TABLE 1: Demographic characteristics and malaria experience of respondents from Bushbuckridge from August 2007 to July 2008.
|
Knowledge of malaria and information, education and communication
The study found that about 93% (95% CI: 90.4–94.7%) of the respondents had heard about malaria, of whom 15.4% (95% CI: 12.5–18.7%)
did not know that malaria is caused by a mosquito bite. The results showed no gender or age specific differences in respondents’ knowledge
of the causes of malaria (Table 2). Notably, respondents (7.5%) who did not know their ages were not far less knowledgeable about malaria causes
than those who knew their ages. All respondents (100%) with tertiary education and 71.8% with no formal education knew that malaria is caused by
mosquito bite (Table 2).
|
TABLE 2: Knowledge of malaria causes stratified by gender, age and the highest level of education attained.
|
The respondents’ sources of malaria information were mainly health facilities (29.1%, 95% CI: 25.5–32.9%) and radio (19.8%, 95% CI:
16.7–23.2%). Malaria communication routes of choice were radio (22.8%, 95% CI: 19.5–26.4%), MCP (14.8%, 95% CI: 12.1–17.9%) and
Community Health Workers (CHWs) (12.5%, 95% CI: 10.0–15.4%). More than one-quarter of the respondents (27.4%) did not mention any preferences,
as shown by ‘not applicable’ (N/A). The proportion of the respondents who preferred community meetings and awareness campaigns to
communicate malaria information, was sizeable (16.4%, 95% CI: 12.4–21.6%), which mirrored characteristics of a close-knit community (Figure 4).
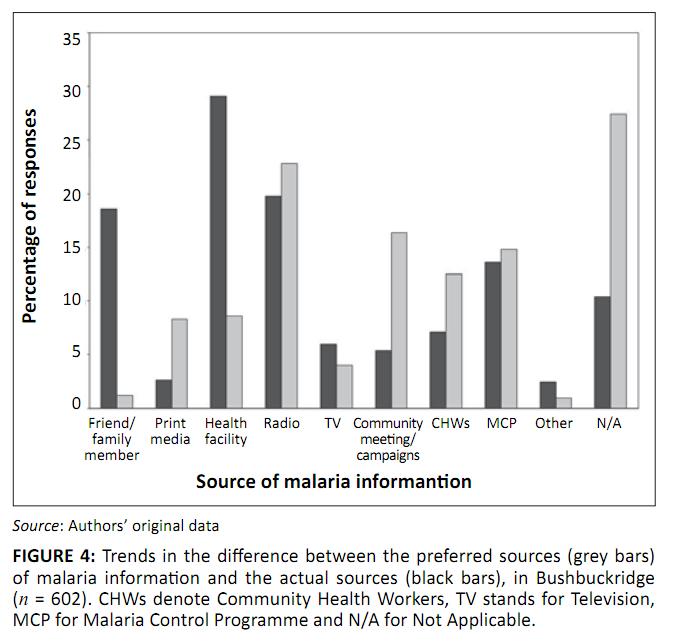 |
FIGURE 4: Trends in the difference between the preferred sources (grey bars)
of malaria information and the actual sources (black bars), in Bushbuckridge
(n = 602). CHWs denote Community Health Workers, TV stands for Television,
MCP for Malaria Control Programme and N/A for Not Applicable.
|
|
Notably, most people (88.2%, 95% CI: 85.3–90.6%) were keen to know more information about malaria, and 90.7% (95% CI: 88.0–92.8%) believed
that malaria can kill if it is untreated. This keenness and attitude towards the fatalistic nature of malaria were interesting findings, especially
when viewed against the background of their limited knowledge of malaria signs and symptoms. About 16.2% could not mention even one sign or symptom
of malaria, whilst 48% only knew one or two signs and symptoms. Few respondents could identify more than four signs or symptoms of malaria (Figure 5).
The most commonly recognised signs and symptoms of malaria (Figure 6) were headache (60%), chills (45%) and high temperature or fever (33%).
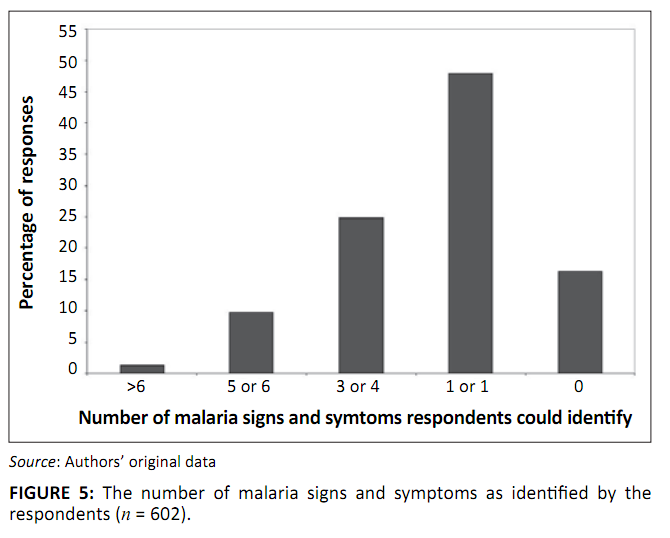 |
FIGURE 5: The number of malaria signs and symptoms as identified by the
respondents (n = 602).
|
|
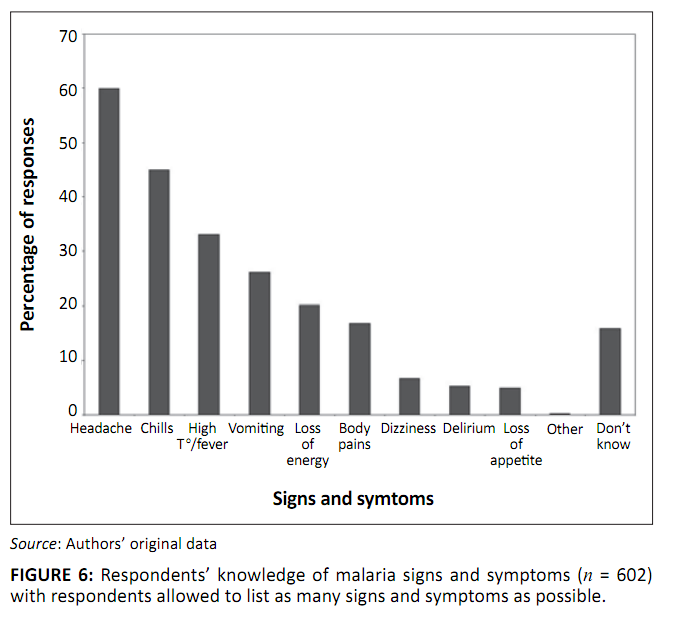 |
FIGURE 6: Respondents’ knowledge of malaria signs and symptoms (n = 602)
with respondents allowed to list as many signs and symptoms as possible.
|
|
Treatment-seeking behaviour and prevention
The study revealed that about 99% (95% CI: 97.5–99.5%) of the respondents preferred to seek treatment at health facilities, 82% of whom
intended to do so within 24 hours of the onset of malaria symptoms. Early treatment-seeking intention amongst women (83.6%) was slightly higher
compared to that amongst men (75.5%); this trend was unaffected by age. In 97% of the time, those who would delay to seek treatment were unlikely
to apply any alternative treatment in the meantime.
About 91% (95% CI: 88.6–93.3%) of the respondents believed that malaria can be prevented through various protective measures. Most respondents
would normally use mosquito coils (51.5%, 95% CI: 47.4–55.5%) and aerosols (35.0%, 95% CI: 31.3–39.0%) to make their houses safer from
mosquitoes, as well as repellents (47.0%, 95% CI: 43.0–51.1%) to avoid mosquito bites (Figure 7). A substantial number of respondents were
unlikely to apply any preventive measures to make their houses safer from mosquitoes (17.3%, 95% CI: 14.4–20.6%) and to avoid mosquito bites
(24.3%, 95% CI: 20.9–27.9%) (Figure 7). Of the 17.3% (n = 104) who would do nothing to make their houses safer from mosquitoes, 75%
(n = 78) reported that their houses had been sprayed within the previous 12 months. About 66% (n = 96) of those who would do nothing
to avoid mosquito bites have had their houses sprayed during the previous spray round. The low use of mosquito nets reflected the non-availability
of a policy on the free distribution of bed nets at all levels in South Africa, that is, at subdistrict, district, provincial and national levels.
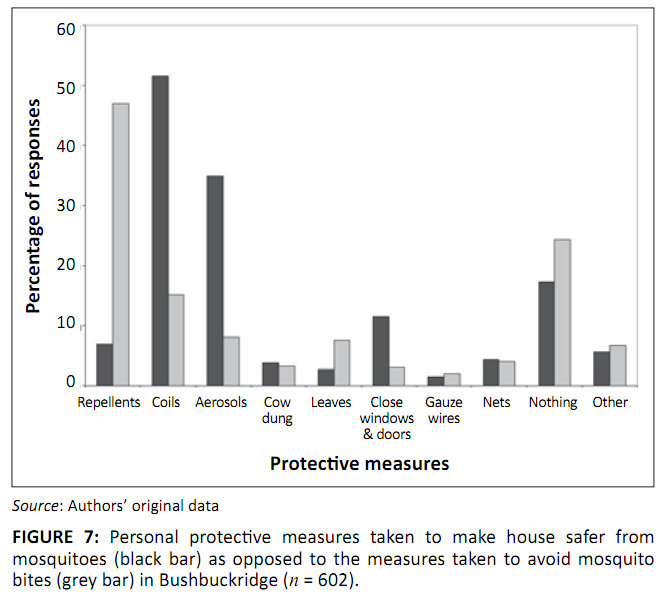 |
FIGURE 7: Personal protective measures taken to make house safer from
mosquitoes (black bar) as opposed to the measures taken to avoid mosquito
bites (grey bar) in Bushbuckridge (n = 602).
|
|
Indoor residual spraying
The results showed that MCP is well known (99.5%, 95% CI: 98.4–99.9%) by the people of Bushbuckridge, who mainly associated its functions
with indoor residual spraying (IRS) activities. About 69.8% (95% CI: 65.9–73.4%) of the respondents reported that their households
had been sprayed, 27.6% (95% CI: 24.1–31.4%) had not been sprayed and 2.7% (95% CI: 1.6–4.4%) did not know whether their households had
been sprayed during the previous spraying season. Re-plastering amongst the 420 (69.8%) sprayed households and 166 (27.6%) unsprayed households was
6.7% and 4.2% respectively (Odds Ratio = 1.62). The two main reasons for nonspraying of certain households were firstly that no one came to spray,
and secondly that no one was at home at the time of spraying. Most respondents (87%, 95% CI: 84.0–89.6%) reported knowledge of the reasons
for spraying and correctly identified the ‘killing of mosquitoes’ as the key reason. However, 29.4% of the respondents did not like
certain aspects about spraying, especially the discolouring of inner house walls by the insecticide (Table 3).
|
TABLE 3: Aspects that people dislike about spraying in Bushbuckridge.
|
Knowledge of malaria
Unlike many malaria-endemic-prone and malaria-epidemic-prone settings in Africa,14,33 appropriate knowledge of the causes of
malaria was relatively good in Bushbuckridge. However, sustaining community knowledge of malaria and its causes may be severely challenged,
and even compromised by the degree of cross-border migration between Mozambique and Bushbuckridge.15,28,29 Studies have noted that
improved community knowledge of malaria and its source of transmission promote preventive and personal protective practices amongst the
affected populations.9,15 Contrary to the results of a study conducted in Tonga, Mpumalanga,34 which established a
close association between knowledge of the causes of malaria and disease symptoms (p < 0.001), no firm relationship between the
knowledge of malaria causes and disease symptoms was identified in this study. The results show that inadequate knowledge of signs and symptoms
in Bushbuckridge does not appear to threaten intentions of prompt treatment-seeking practices at this stage. However, if no attention is paid to
educating the community on malaria signs and symptoms, the future implications cannot be guaranteed because other studies have established that
the knowledge of signs and symptoms plays a particular role on early diagnosis and treatment.35
Malaria information, education and communication
Congruent with other findings,2,36 this study identified radio, as the medium for spreading information speedily and widely, as one
of the most commonly used malaria information communication routes. The number of respondents who preferred CHWs and MCP to communicate malaria
information was noteworthy if it is taken into account that CHWs have been found to be effective in delivering messages on specific control,
treatment and prevention behaviours on individual bases.39 The use of community meetings and awareness campaigns in this study is
indicative of the strength and value of involving community leadership and structures as strategic partners in malaria-control activities. Without
community consent and cooperation, for example, successful implementation of interventions such as IRS will be an illusion. It is, therefore,
necessary to ensure that locally acceptable and appropriate communication routes are used for information, education and communication (IEC)
interventions. There are serious concerns however, about the fact that 27.4% did not specify any preferred information communication routes,
and this finding requires further investigation. Despite mixed responses on the respondents’ preferred malaria information communication
routes, their attitude and keenness to receive more information about malaria was positive.
Treatment-seeking practices in terms of promptness and treatment options
Respondents’ intentions to seek treatment promptly and their choice of a treatment facility in Bushbuckridge exceeded the Roll Back Malaria
(RBM) recommendation, which states that at least 80% of those infected with malaria should seek prompt (within 24 hours of the onset of symptoms)
treatment.21 Similarly, the Lubombo Spatial Development Initiative (LSDI) region, a collaborative approach to malaria control between
Mozambique, Swaziland and South Africa, was found to have high prompt treatment-seeking habits.38,39 This trend was contrary to many
African countries13,14,40,41,42 whereby nonpublic health facilities are mostly preferred and promptness remained as low as
13–30%.14,40,42 Treatment-seeking trends in LSDI were accounted for by the effectiveness of country MCPs and the project
successes.43 The inhabitants’ willingness to seek treatment promptly in Bushbuckridge was explained furthermore by their attitude
towards malaria, whereby over 90% thought that malaria can be deadly if left untreated. It should be noted that a positive attitude is particularly
important when attempting to change behaviour.
Personal protection against malaria and indoor residual spraying
Most respondents in this study correctly distinguished between measures intended to make a house safer and those intended to avoid mosquito bites.
Of more concern was the fact that between 17% and 25% of the people did not use any personal protection against malaria. On a somewhat positive note,
66–75% of those who did not use any personal protection were protected by IRS, as they reported to have had their houses sprayed during the
previous spraying round. However, the remaining 25–34% whose houses were not sprayed and yet did not use any personal protection was equally
concerning. Mosquito net ownership and use was low, probably because South Africa does not have a mosquito nets distribution programme. The results
of this study suggest that IRS coverage still need some improvement, especially as WHO recommends a minimum of 80% spraying coverage for IRS to be
effective.32 Re-plastering as a common social practice identified by many studies to compromise the residual efficacy of the
insecticide,44 was relatively low in Bushbuckridge, whereas the practice was found to be a serious threat in the study conducted in
KwaZulu-Natal, South Africa.45
It is very interesting that this study has shown that re-plastering in Bushbuckridge is often undertaken to prepare for the festive season, as opposed
to the dominant thinking that people solely re-plaster to remove insecticide marks on the house wall.45 Even more interesting was the fact
that the proportion of householders who re-plastered after spraying did not enormously differ from those whose households were not sprayed but
re-plastered anyway. This finding suggests that there is more to re-plastering than just a reaction to insecticide marks on the walls; hence
interventions directed at discouraging re-plastering should take this factor into consideration and should probably advise the community on the
acceptable time of the year to re-plaster their houses. This recommendation takes into consideration the fact that it may be an ambitious view to
believe that re-plastering could cease completely.
In conclusion, most respondents had a fair knowledge of malaria and its transmission. Educating communities on malaria signs and symptoms may
need some attention before it negatively affects treatment-seeking behaviour. In line with WHO recommendation, IRS coverage needs some improvements,
especially because it remains the mainstay of vector control intervention in Bushbuckridge and other malarious areas in South Africa. It is apparent
that a multiplicity of factors are involved in the decision to re-plaster which suggests that new strategies and approaches are required to address
the impact this practice has on the effectiveness of malaria control efforts. The results of this study displayed generally acceptable community
awareness on effectiveness of malaria control efforts. However, the fact that the study did not investigate the respondents’ origins and their
migratory history is considered a limitation, especially given the degree of cross-border movement between Mozambique and Bushbuckridge. The impact
of migration on malaria in Bushbuckridge was not adequately explored either.
The authors gratefully acknowledge data collectors and Malaria Control Programme staff in Bushbuckridge, Mpumalanga Province, for all their
logistical support. The authors also sincerely thank the Database and GIS Sections of the Malaria Research Programme of the Medical Research
Council for all their technical assistance, which ranged from data capturing and cleaning to data mapping. The study was financially supported
by the authors’ organisations.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
K.W.H. was a project leader and participated in all the research processes. A.Z. and A.M.M. were involved in data collection, its analysis and
the interpretation of the results. RM made conceptual contributions as well as meaningful inputs in the development of a manuscript.
1. Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in Southern Africa with emphasis on the use of indoor residual
house-spraying. Trop Med Int Health. 2004;9(8):846–856.
2. Joshi AB, Banjara MR. Malaria related knowledge, practices and behaviour of people in Nepal. J Vector Borne Diseases. 2008;45:44–50.
PMid:18399316
3. Onwujekwe OE, Akpala CO, Ghasi S, Shu EN, Okonkwo PO. How do rural households perceive and prioritise malaria and mosquito nets? A study
in five communities of Nigeria. Public Health. 2000;114(5):407–410.
doi:10.1016/S0033-3506(00)00373-5
4. Rugemalila JB, Ogundahunsi OAT, Stedman TT, Kilama WL. Multilateral Initiative on Malaria: Justification, evolution, achievements, challenges,
opportunities, and future plans. Am J Trop Med Hyg. 2007;77(Suppl. 6):296–302.
PMid:18165506
5. Snow RW, Graig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant
population. Bull World Health Organ. 1999;77(8):624–640.
PMid:10516785,
PMid:2557714
6. Borah PK, Dutta P, Phukan AC, Mahanta J. Health seeking behaviour among the fever cases during an outbreak of malaria in Assam.
J Hum Ecol. 2004;15(3):195–197.
7. Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: What’s new, what’s needed: A summary. Am J Trop
Med Hyg. 2004;71(Suppl. 2):6–11.
8. Agyepong IA, Kangeya-Kayonda J. Providing practical estimates of malaria burden for health planners in resource-poor countries. Am J Trop Med Hyg.
2004; 71(Suppl.2):162−167.
PMid:15331833
9. Ahorlu CK, Koram KA, Arholu C, De Savigny D, Weiss MG. Socio-cultural determinants of treatment delay for childhood malaria in southern
Ghana. Trop Med Int Health. 2006;11(7):1022−1031.
doi:10.1111/j.1365-3156.2006.01660.x
10. De Savigny D, Mayombana C, Mwageni E, et al. Care-seeking pattern for fatal malaria in Tanzania. Malar J. 2004; 3:27.
doi:10.1186/1475-2875-3-27,
PMid:15282029,
PMid:514497
11. Goodman CA, Mills AJ. Review article: The evidence base on the cost-effectiveness of malaria control measures in Africa. Health Policy Plan.
1999;14(4):301−312. doi:10.1093/heapol/14.4.301,
PMid:10787646
12. Macintyre K, Keating J, Sosler S, et al. Examining the determinants of mosquito-avoidance practices in two Kenyan cities. Malar J. 2002;1:14.
doi:10.1186/1475-2875-1-14,
PMid:12495438,
PMid:149385
13. Müller O, Traoré C, Becher H, Kouyaté B. Malaria morbidity, treatment-seeking behaviour, and mortality in a cohort of young children
in rural Burkina Faso. Trop Med Int Health. 2003;8(4):290−296.
14. Deressa W, Ali A, Enquoselassie F. Knowledge, attitudes and practices about malaria, the mosquito and antimalarial drugs in a rural community.
Ethiop J Health Dev. 2003;17(2):99–104.
15. Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proc Natl Acad Sci.
2010;107(27):12222–12227.
doi:10.1073/pnas.1002971107,
PMid:20566870,
PMid:2901446
16. Martens P, Hall L. Malaria on the move: Human population movement and malaria transmission. Emerg Infect Dis. 2000;6(2):103–109.
doi:10.3201/eid0602.000202,
PMid:10756143,
PMid:2640853
17. Tyagi P, Roy A, Malhotra MS. Knowledge, awareness and practices towards malaria in communities of rural, semi-rural and bordering areas of
east Delhi (India). J Vector Borne Diseases. 2005;42(1):30–35.
PMid:15999459
18. Barnes KI, Durrheim DN, Little F, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in
KwaZulu-Natal, South Africa. PLoS Medicine. 2005;2(11):1123–1134.
19. Sharp BL, Le Sueur D. Malaria in South Africa–The past, the present and selected implications for the future. S Afr Med J.
1996;86(1):83–89.
PMid:8685790
20. Department of Health. Draft South Africa communication strategy for malaria elimination; August 2010 (Unpublished).
21. World Health Organisation. World Malaria Report. Geneva: Switzerland; 2008.
22. Coleman M, Coleman M, Mabuza AM, Kok G, Coetzee M, Durrheim D. Evaluation of an operational malaria outbreak identification and response
system in Mpumalanga Province, South Africa. Malaria J. 2008;7:69.
doi:10.1186/1475-2875-7-69,
PMid:18439307,
PMid:2405804
23. Statistics South Africa. Census 2001: Primary Tables South Africa Census 1996 and 2001 Compared. Government Publications Department Report No. 03-02-04; 2004.
24. Map developed by GIS Laboratory of the Malaria Research Unit, MRC.
25. Pérez de Mendiguren JC. Productive uses of water at household level: Evidence from Bushbuckridge. Paper presented at: International
Symposium on Water, Poverty and Productive Uses of Water at the Household Level; 2003 Jan 21–23; Muldersdrift, South Africa. p. 57–72.
26. Tollman SM. The Agincourt field site–Evolution and current status. S Afr Med J. 1999;89(8):853–858.
PMid:10488361
27. Yeatman L, Du Toit M, Andreasen L. Developing approaches for improved natural woodland resource use and management: Lessons learnt in the
communal areas of Bushbuckridge (Part 2). DWAF, South Africa. 2003.
28. Polzer T. Adapting to changing legal frameworks: Mozambican refugees in South Africa. Int J Refugee Law. 2007;19(1):22–50.
doi:10.1093/ijrl/eem001
29. Polzer T. Invisible integration: How bureaucratic, academic and social categories obscure integrated refugees. J Refugee Stud. 2008;21(4):476–498.
doi:10.1093/jrs/fen038
30. MCP Manager’s presentation at LSDI meeting in August 2010.
31. Roll Back Malaria Partnership. The Global Malaria Action Plan. Roll Back Malaria Partnership; 2008.
32. World Health Organisation. Implementation of indoor residual spraying of insecticides for malaria control in the WHO African Region.
Vector Biology and Control Unit, Division of Healthy Environments and Sustainable Development: WHO Africa; 2007.
33. Munguti KJ. Community perceptions and treatment seeking for malaria in Baringo district, Kenya: Implications for disease control. East
Afr Med J. 1998;75(12):687–691.
PMid:10065206
34. Govere J, Durrheim D, La Grange K, Mabuza A, Booman M. Community knowledge and perceptions about malaria and practices influencing
malaria control in Mpumalanga Province, South Africa. S Afr Med J. 2000;90(6):611–615.
PMid:10918892
35. Nkuo-Akenji TK, Ntonifor NN, Ching JK, et al. Evaluating a malaria intervention strategy using knowledge, practices and coverage surveys
in rural Bolifamba, south-west Cameroon. Trans R Soc Trop Med Hyg. 2005;99(5):325–332.
doi:10.1016/j.trstmh.2003.12.016,
PMid:15780338
36. Ndyomugyenyi R, Magnussen P, Clarke S. Malaria treatment-seeking behaviour and drug prescription practices in an area of low
transmission in Uganda: Implications for prevention and control. Trans R Soc Trop Med Hyg. 2007;101(3):209–215.
doi:10.1016/j.trstmh.2006.06.004,
PMid:16950487
37. Cropley L. The effect of health education interventions on child malaria treatment-seeking practices among mothers in rural refugee
villages in Belize, Central America. Health Promot Internation. 2004;19(4):445–452.
doi:10.1093/heapro/dah406,
PMid:15520038
38. Castillo-Riquelme M, McIntyre D, Barnes K. Household burden of malaria in South Africa and Mozambique: Is there a catastrophic impact?
Trop Med Int Health. 2008;13(1):108–122.
doi:10.1111/j.1365-3156.2007.01979.x
39. Hlongwana KW, Mabaso MLH, Kunene S, Govender D, Maharaj R. Community knowledge, attitudes and practices (KAP) on malaria in Swaziland:
A country earmarked for malaria elimination. Malar J. 2009;8:29.
doi:10.1186/1475-2875-8-29,
PMid:19228387
PMid:2649151
40. Dunyo SK, Afari EA, Koram KA, Ahorlu CK, Abubakar I, Nkrumah FK. Health centre versus home presumptive diagnosis of malaria in
southern Ghana: Implications for home-based care policy. Trans R Soc Trop Med Hyg. 2000;94(3):285–288.
doi:10.1016/S0035-9203(00)90324-9
41. Nuwaha F. People’s perception of malaria in Mbarara, Uganda. Trop Med Int Health. 2002;7(5):462–470.
doi:10.1046/j.1365-3156.2002.00877.x
42. Kilian AHD, Tindyebwa D, Gülck T, et al. Attitude of women in Western Uganda towards pre-packed, unit-dosed malaria treatment for children.
Trop Med Int Health. 2003;8(5):431–438.
doi:10.1046/j.1365-3156.2003.01044.x
43. Sharp BL, Kleinschmidt I, Streat E, et al. Seven years of regional malaria control collaboration–Mozambique, South Africa, and Swaziland.
Am J Trop Med Hyg. 2007;76(1):42–47.
PMid:17255227
44. Mnzava AEP, le Sueur D, Sharp B, Mthembu JD, Ntuli VM. Opinion. Malaria in the new South Africa–are district health systems and current
malaria control strategies compatible? S Afr Med J. 1997;87(5):585–587.
PMid:9254813
45. Mnzava AEP, Sharp B, Mthembu JD, et al. Malaria control–Two years’ use of insecticide-treated bednets compared with insecticide
house spraying in KwaZulu-Natal. S Afr Med J. 2001;91(11):978–983.
PMid:11847921
|
|

